Candida albicans is a naturally occurring yeast that lives in the mouth, gut, skin, and genital tract of healthy individuals. In most people, it coexists harmlessly with beneficial bacteria as part of a balanced microbiome [1][2][3]. When this internal environment becomes disrupted, Candida can increase in number – particularly within the digestive tract – leading to symptoms often described as Candida overgrowth. This reflects a localised gut imbalance, not the systemic infections seen in hospital settings.
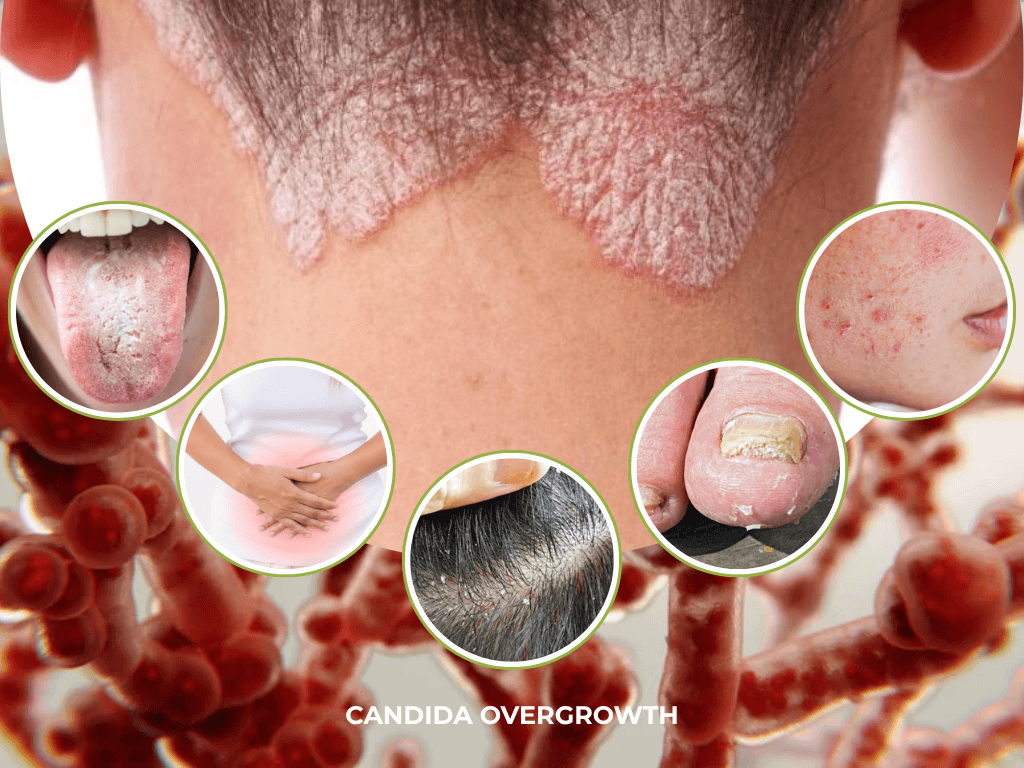
Often, this condition first appears as localised infections [1]. In these cases, classic symptoms include white patches, redness, discomfort, and itching [4]. However, persistent or systemic overgrowth may contribute to a broader constellation of health complaints.
Older adults are typically at heightened risk of severe Candida infections [5][6]. In fact, ageing, medication use, reduced stomach acid, and changes in gut immunity can shift the internal environment in ways that allow yeasts to flourish more easily.
Several compounding risk factors contribute to this vulnerability, including chronic pulmonary and cardiovascular disease, diabetes mellitus, and renal failure [7]. The normal ageing process itself – involving waning immune defences, changes in oral health, and increased likelihood of nutritional deficiencies – makes older adults more susceptible.
Understanding Candida Overgrowth and its Associated Risks
Candida levels in the gut tend to rise when the delicate balance of the intestinal ecosystem is disrupted in ways that favour yeast over beneficial bacteria.
Under healthy conditions, a diverse microbiome, intact mucosal immunity, and robust digestive function work together to keep Candida in a controlled, commensal state. When diet, medications, stress, or immune changes shift this balance, Candida can more easily proliferate and contribute to a range of gut and systemic symptoms.
In this way, Candida species can shift from harmless commensals to pathogenic organisms.
High-sugar and refined-carbohydrate diets are a key driver of this shift because they provide readily fermentable fuel that Candida can use to grow and adhere to mucosal surfaces [8]. Frequent intake of sugary foods or drinks and ultra-processed carbohydrates is associated with dysbiosis, encouraging opportunistic fungi while undermining microbial diversity and metabolic stability. At the same time, these diets can promote blood sugar fluctuations and cravings, creating a cycle in which ongoing high sugar intake continues to support yeast overgrowth.
Antibiotic exposure is another common trigger, because broad-spectrum antibiotics reduce protective commensal bacteria that normally compete with Candida for nutrients and attachment sites, and help maintain pH and short-chain fatty acid production [9]. With these bacterial populations suppressed, Candida encounters fewer checks and balances and can expand into ecological niches that would otherwise be occupied by beneficial microbes [9].
Changes in digestive chemistry can also play a significant role. Altered bile acid composition, low stomach acid, or inadequate pancreatic and bile secretions modify the pH and detergent-like effects that normally help regulate microbial populations in the upper gut [10]. Certain clinical evidence indicates that shifts in bile acids can influence Candida’s morphology and colonisation patterns, making it easier for the organism to persist [10]. At the same time, lower levels of mucosal immune markers such as secretory IgA mean that microbes are less effectively ‘coated’ and contained at the gut surface, allowing greater fungal adherence.
Also of note, emerging research also indicates that Candida overgrowth may be influenced by host genetics (a person’s inherited traits that affect immune response), epithelial integrity (the strength of the body’s protective cell lining), and metabolic factors [11]. For instance, altered bile acid composition, changes in gut pH, or deficiencies in secretory IgA (a protective antibody that protects mucosal surfaces like the gut and respiratory tract) can all affect yeast behaviour within the gastrointestinal tract [12].
While a brief Candida bloom is often benign, persistent overgrowth may contribute to a spectrum of health complaints – including digestive discomfort (bloating, gas, bowel disturbances), chronic fatigue, ‘brain fog’, mood changes, and frequent infections – particularly when microbiome imbalance is present, or immunity is compromised [1] [13]. Some individuals also report skin issues, sugar cravings, and feelings of general malaise [14], further indicating how pervasive and disruptive candidiasis can be when left unchecked.
Recognising the Role of Nutrition and Lifestyle
In recent years, the conversation around Candida has moved beyond treating simple, local infections to addressing whole-body root causes – microbiome imbalance, nutrition, stress resilience, and lifestyle. Scientists are increasingly investigating the complex interplay between gut flora, immune function, and metabolic health, aiming to identify targeted strategies that go beyond symptom management.
There is now a greater emphasis on restoring microbial harmony, repairing gut health, and supporting immune function, spotlighting the importance of dietary choices and lifestyle adjustments as central to long-term management and recovery. Because the end goal is not to eliminate Candida (it is a normal resident), but to restore microbial harmony.
Research has shown that diets high in refined carbohydrates, sugars, and ultra-processed foods may promote the overgrowth of Candida, as these organisms thrive on simple sugars and can outcompete beneficial bacteria under such conditions [15]. One example of this is a study evidencing that high glucose levels are shown to stimulate Candida growth by up to 12-fold within hours, creating an environment that favours fungal proliferation over beneficial bacteria [16].
Conversely, nutrient-dense, fibre-rich diets that include fermented foods, prebiotics, and probiotics have been associated with a more balanced gut microbiome and reduced Candida colonisation. For instance, probiotics containing Lactobacillus and Bifidobacterium strains have demonstrated the ability to inhibit Candida adhesion to mucosal surfaces and restore microbial equilibrium [17].
Demonstrating the influence of diet, a pilot study involving 120 patients with chronic intestinal Candida overgrowth found that 85% of those who adhered to a recommended diet modification alongside conventional antifungal treatment were cured after three months, showing no Candida growth or symptoms. In contrast, only 42.5% of patients treated with antifungals alone, without dietary changes, achieved the same outcome [18].
Lifestyle and immune function are also central to understanding Candida susceptibility. Chronic stress, inadequate sleep, and prolonged use of antibiotics or corticosteroids can weaken immune defences and disrupt microbial balance, making the body more vulnerable to opportunistic infections [14][19].
Studies on stress-related immune modulation reveal that elevated cortisol levels can suppress the activity of key immune cells involved in fungal defence, such as neutrophils and macrophages. Therefore, holistic management of Candida-related conditions increasingly incorporates stress reduction techniques – such as mindfulness, yoga, and regular physical activity – alongside medical treatment to enhance resilience and restore systemic balance.
Applying Nutritionist-Led Dietary Strategies and Lifestyle Adjustments
This integrated approach – combining evidence-based medical treatment with targeted nutritional and lifestyle strategies – represents a paradigm shift toward more sustainable and comprehensive care for Candida-related health concerns. Rather than relying solely on antifungal medications, this approach recognises the importance of addressing underlying dietary and lifestyle factors that contribute to yeast overgrowth.
A nutritionist-led plan typically focuses on a low-sugar, anti-inflammatory diet that reduces refined carbohydrates and processed foods – prime fuels for Candida. Consuming plenty of non-starchy vegetables, healthy proteins and fats, fermented foods, and low-sugar fruits supports a balanced gut microbiome and helps curb yeast proliferation.
Beyond this, avoidance of added sugars, excessive alcohol, and caffeine helps create an internal environment less favourable to Candida growth. Lifestyle habits such as managing stress, getting adequate sleep, staying well-hydrated, and incorporating gentle exercise further reinforce immune health and digestive balance.
This more sustainable paradigm of care that treats the whole person and integrates various therapeutic angles. Looking beyond temporary symptom suppression not only helps to curb Candida proliferation but also supports overall digestive health, potentially reducing the risk or severity of conditions like diverticulitis, which can flare when gut flora is imbalanced.
Tackling Candida Overgrowth With The Health Suite
At The Health Suite, a range of carefully selected advanced functional and medical tests can be used, which can help clarify whether Candida is contributing to symptoms, when appropriate, to build a clearer picture of internal balance and guide targeted nutrition and lifestyle strategies to best support overall gut and immune health.
For example, comprehensive stool microbiome testing can assess Candida species alongside beneficial bacteria, short-chain fatty acid production, gut inflammation, bile acids, and overall microbial diversity, providing a detailed view of the gastrointestinal ecosystem. This level of insight helps to identify whether yeast overgrowth is present in the context of broader dysbiosis, impaired digestion, or inflammatory activity.
For some individuals, an organic acids test (OAT) offers further information about systemic physiology by measuring urinary metabolites. This may include yeast-related compounds such as D-arabinitol, as well as markers linked to mitochondrial function, detoxification capacity, and key vitamin or nutrient insufficiencies. By highlighting biochemical imbalances that often accompany chronic gut issues, OAT results can help refine supplement choices, dietary adjustments, and lifestyle recommendations.
Alongside functional checks, blood tests can play an important role in assessing factors that influence Candida behaviour and host resilience. Measures such as fasting glucose and HbA1c are relevant because chronically elevated blood sugar can provide a more favourable environment for yeast proliferation, while vitamin D, B vitamins, and inflammatory markers like CRP help to evaluate immune competence and systemic inflammation. Sometimes, additional markers of gut barrier and immune function, such as secretory IgA or zonulin, may offer further insight into mucosal integrity and local defence at the intestinal lining.
But importantly, testing is always optional and is used to complement, rather than replace, a thorough clinical assessment.
When chosen, these investigations allow The Health Suite team to personalise functional medicine and nutrition-based treatment plans with greater precision, aligning dietary interventions, probiotic support, and lifestyle recommendations with each person’s unique microbiome, metabolic profile, and immune status.
This integrative, data-informed approach helps move beyond symptom suppression towards restoring microbial balance, supporting gut barrier health, and promoting more sustainable wellbeing over the long term.
Start your journey to a balanced microbiome and more sustainable wellbeing with The Health Suite’s personalised approach to Candida Overgrowth with our Nutritional Therapy Services. and Functional Medicone Tests.
Support your microbiome and wellbeing with personalised Candida care.
References:
- R AN, Rafiq NB. Candidiasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available at: https://www.ncbi.nlm.nih.gov/books/NBK560624/
- Talapko J, et al. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J Fungi (Basel). 2021; 22;7(2):79
- Healthline. 6 Symptoms of Candida Overgrowth and Tips to Get Rid of It. Available at: https://www.healthline.com/nutrition/candida-symptoms-treatment
- CDC. Symptoms of Candidiasis. Available at: https://www.cdc.gov/candidiasis/signs-symptoms/index.html
- UK HSA. Research and analysis. Bloodstream infection due to Candida (and species formerly part of the Candida genus) in England: 2022. Available at: https://www.gov.uk/government/publications/candidaemia-annual-data-from-voluntary-surveillance/bloodstream-infection-due-to-candida-and-species-formerly-part-of-the-candida-genus-in-england-2022
- Dekkers BGJ, et al. Invasive Candidiasis in the Elderly: Considerations for Drug Therapy. Drugs Aging. 2018 Sep;35(9):781-789
- Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in Patients with Diabetes Mellitus. J Clin Med. 2019; 10;8(1):76
- Kumamoto CA, Gresnigt MS, Hube B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr Opin Microbiol. 2020;56:7-15
- d’Enfert C, et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives. FEMS Microbiol Rev. 2025;45(3):fuaa060
- Jacobsen ID. The Role of Host and Fungal Factors in the Commensal-to-Pathogen Transition of Candida albicans. Curr Clin Microbiol Rep. 2023;10(2):55-65
- Brown AJ, et al. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22(11):614-22
- Bamba S, et al. Relationship between the gut microbiota and bile acid composition in the ileal mucosa of Crohn’s disease. Intest Res. 2022;20(3):370-380
- Dublin Centre for Functioning Medicine. Understanding the Link Between Candida and Chronic Fatigue Syndrome. Available at: https://dublincfm.com/digestive-health/candida/understanding-the-link-between-candida-and-chronic-fatigue-syndrome/#:~:text=Candida%20overgrowth%2C%20also%20known%20as,bloodstream%2C%20heart%2C%20and%20brain.
- Holland & Barrett. Candida: Symptoms, treatments & causes – explained. Available at: https://www.hollandandbarrett.com/the-health-hub/conditions/womens-health/candida/what-is-candida/
- Jawhara S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms. 2023; 11;11(6):1556
- Man A, et al. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem Inst Oswaldo Cruz. 2017;112(9):587-592
- Song J, et al. Gut bacteria: protective mediators, pathogenic contributors and novel therapeutic targets in Candida albicans infections. Gut Pathog. 2025; 2;17(1):77
- Otašević, S, et al. The dietary modification and treatment of intestinal Candida overgrowth – a pilot study. Journal de Mycologie Médicale. 2018; 28(4), 623-627
- Warren A, et al. Dangers of the chronic stress response in the context of the microbiota-gut-immune-brain axis and mental health: a narrative review. Front Immunol. 2024; 2;15:1365871
